IT’S TIME FOR EXPANDED NIPT
EXPANDED SCREENING WITH UNPARALLELED ACCURACY
NIPT now endorsed by ACOG/SMFM for all pregnant mothers regardless of age or risk
Also known as cell-free DNA (cfDNA) screening,noninvasive prenatal testing (NIPT) is a prenatal aneuploidy screening test.
Can noninvasively screen for the presence of fetal chromosomal aneuploidies as early as week 10.
WHAT WILL YOU GAIN
WITH AN EXPANDED NIPT SCREEN?
MORE INSIGHTS.
Fetal chromosome abnormalities aren’t limited to 3 common trisomies. Why should your screening be?
Prenatal screening for trisomies 21, 18, and 13 has been available for over 30 years. The narrow focus on these 3 aneuploidies was due to test limitations, not because they were the only known chromosomal abnormalities.
With cell-free DNA (cfDNA)–based prenatal testing, also called noninvasive prenatal testing (NIPT), we can use expanded screening to obtain a comprehensive view of all 23 chromosome pairs while limiting the risk to your patient.

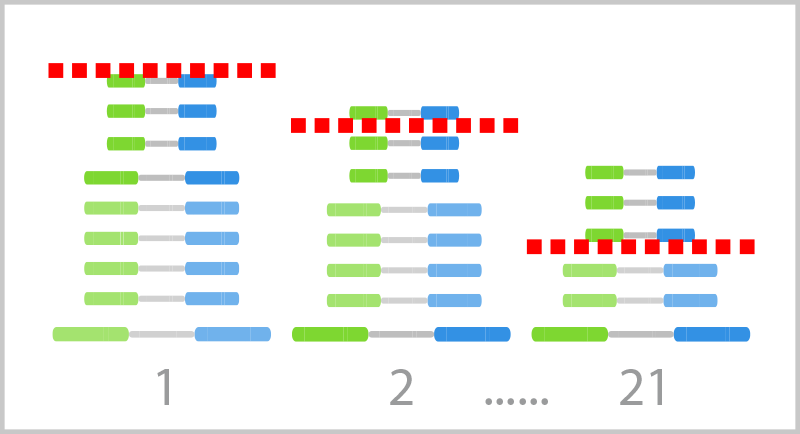
NIPT24 OVERVIEW
The NIPT24 (Illumina Veriseq NIPT Solution V2) is an in vitro diagnostic (IVD) test intended for use as a screening test for the detection of genome-wide fetal genetic anomalies from maternal peripheral whole blood specimens in pregnant women of at least 10 weeks gestation. NIPT24 uses Whole-Genome Sequencing(WGS) to detect partial duplications and deletions for all autosomes and aneuploidy status for all chromosomes. The test offers an option to request the reporting of Sex Chromosome Aneuploidy (SCA). This product must not be used as the sole basis for diagnosis or other pregnancy management decisions.
NonInvasive Prenatal Testing (NIPT) based on Cell-free DNA analysis from maternal blood is a screening test; it is not diagnostic. False positive and false negative results do occur. Test results must not be used as the sole basis for diagnosis. Further confirmatory testing is necessary prior to making any irreversible pregnancy decision. A negative result does not eliminate the possibility that the pregnancy has a chromosomal or sub chromosomal abnormality. This test does not screen for polyploidy (eg, triploidy), birth defects such as open neural tube defects, single gene disorders, or other conditions, such as autism. There is a small possibility that the test results might not reflect the chromosomal status of the fetus, but may instead reflect chromosomal changes in the placenta (Confined Placental Mosaicism, CPM) or the mother that may or may not have clinical significance.
THE SCIENCE BEHIND NIPT24

Sample Prep
First, plasma is isolated from whole blood for cfDNA extraction.
Then, utilizing all available cfDNA, the whole-genome and PCR-free approach, maximizes efficiency and robustness of the assay, as demonstrated by the lowest failure rate among in-lab solutions.
Lastly, uniquely tagging the fragments from each sample using adapters

Sequencing
The ends of each fragment are sequenced using 2X36 bp reads.
These paired-end reads are aligned to the genome, contextually providing the length of each fragment (measured by the distance between the two paired reads).
Fragment length is valuable because shorter cfDNA fragments are more likely to be fetal in origin, while longer cfDNA fragments are more likely to be maternal in origin.
Analysis and Reporting
A dynamic threshold individually assesses the quality and quantity of the collected reads for each sample to determine if there is enough information to make a call.
If so, these reads are sorted by chromosomes to look for deviations from the expected distribution.
By focusing on shorter fragments, there is an increase in relative fetal signal, allowing excellent results without a fetal fraction cutoff.
GAIN EXPANDED NIPT INSIGHTS
NIPT24 gives you valuable information about the chromosomal status of the fetus as early as 10 weeks’ gestation. Newer tests take advantage of next-generation sequencing (NGS) to bring a whole-genome sequencing (WGS) approach to NIPT, expanding test options beyond chromosomes 21, 18, and 13 to include rare autosomal aneuploidies (RAAs), sex chromosome aneuploidies (SCAs),
and partial deletions and duplications, also referred to as copy number variations (CNVs), ≥7 Mb in size. Traditional, targeted tests do not screen for these additional chromosomal abnormalities, which have been associated with clinically relevant outcomes such as developmental delays, intellectual disabilities, structural anomalies, and adverse pregnancy outcomes.
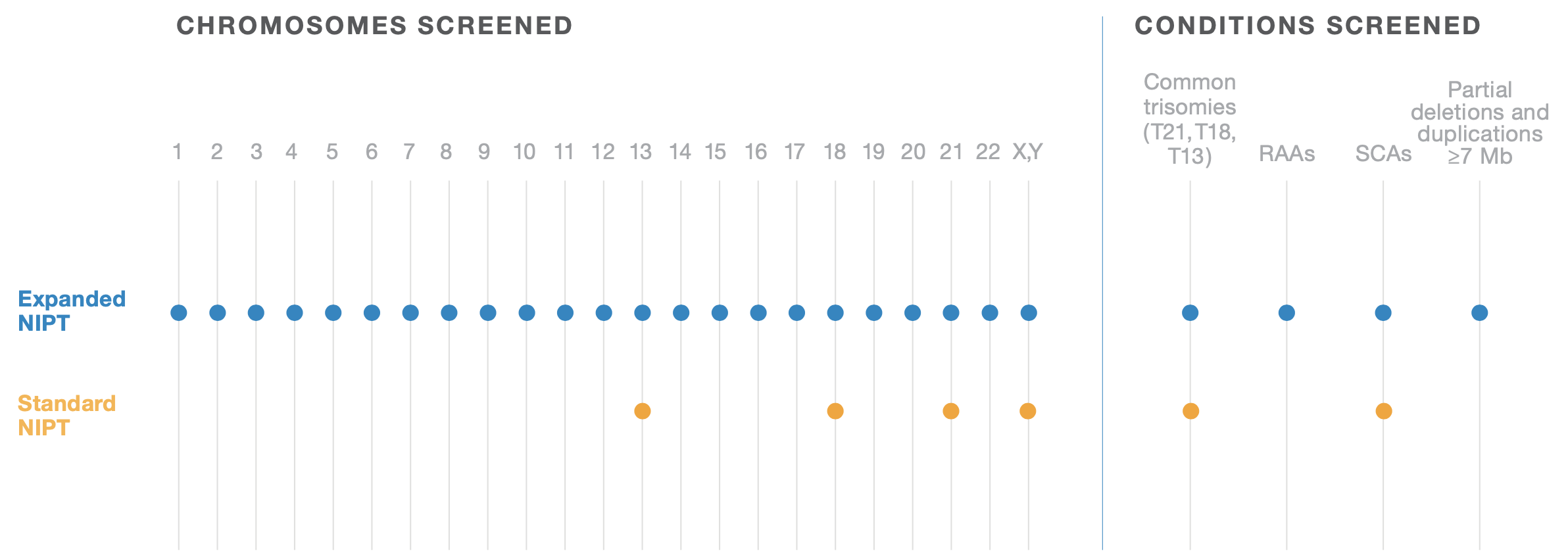
RARE IS MORE COMMON THAN YOU THINK
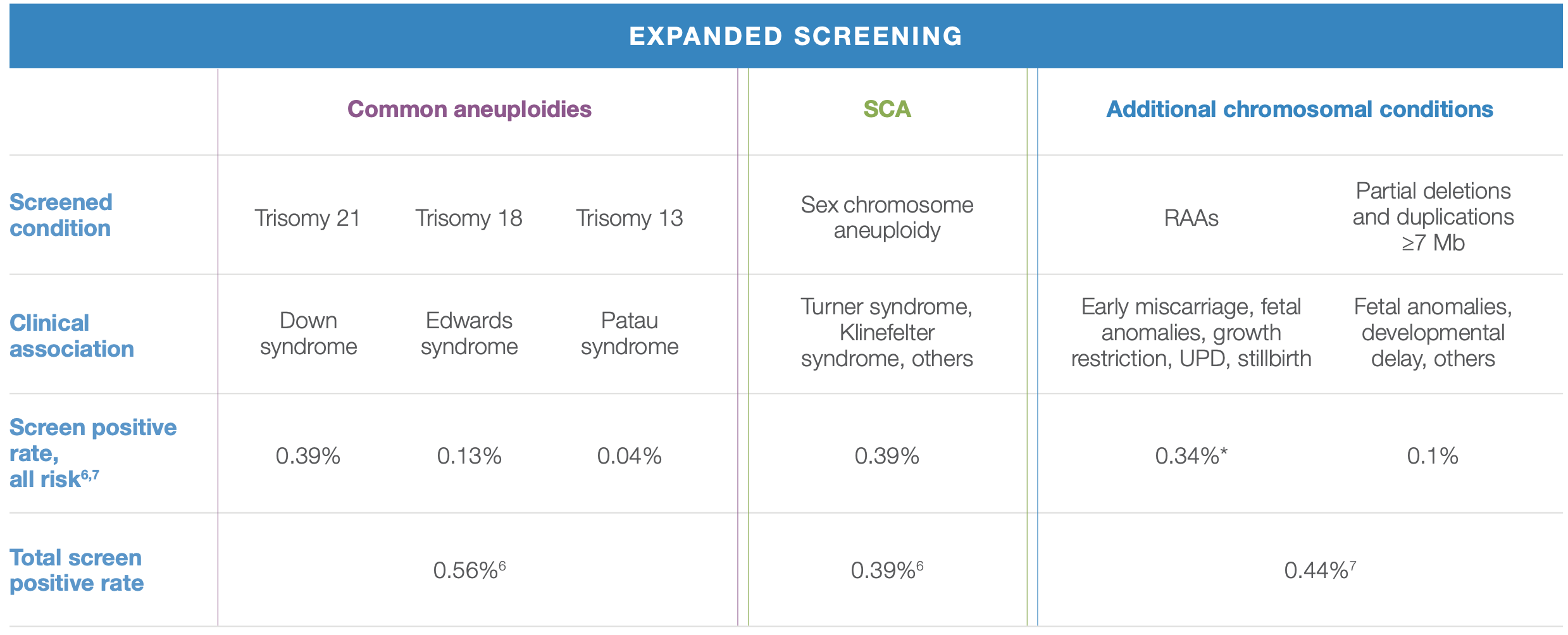
While called rare, rare autosomal aneuploidies (RAAs) are more prevalent than you may expect. Their combined screen positive rate in early pregnancy is estimated to be 0.44%.
Compare that to the estimated combined 0.56% screen positive rate of trisomies 21, 18, and 13. This means that you may be missing something if you’re not looking at the whole genome.
WHY EXPANDED NIPT SCREENING?
WHY RAAs AND CNVs MATTER
A positive RAA or CNV NIPT result can represent the fetal and/or placental genome. Either way, a positive result indicates an increased risk for fetoplacental disease and is associated with clinical relevance in half of the cases. Even if the RAA or CNV is mosaic in nature, an abnormality within the fetus and/or placenta may manifest itself.
Possible implications of RAA- or CNV-positive results include:

Risk of miscarriage—early screening may help in understanding the cause of a miscarriage and often reassures about the recurrence risk for a future pregnancy with a chromosomal abnormality.

Risk of fetal anomalies—RAAs and CNVs may have an adverse effect on organ and brain development, resulting in structural anomalies, developmental delays, and intellectual disability; even if the RAA is only present in the placenta, uniparental disomy in the fetus can lead to adverse effects on development.

Risk of adverse outcomes—if the RAA or CNV is confined to the placenta, the fetus may be chromosomally normal, but problems with the placenta may result in poor fetal growth and/or preterm labor.
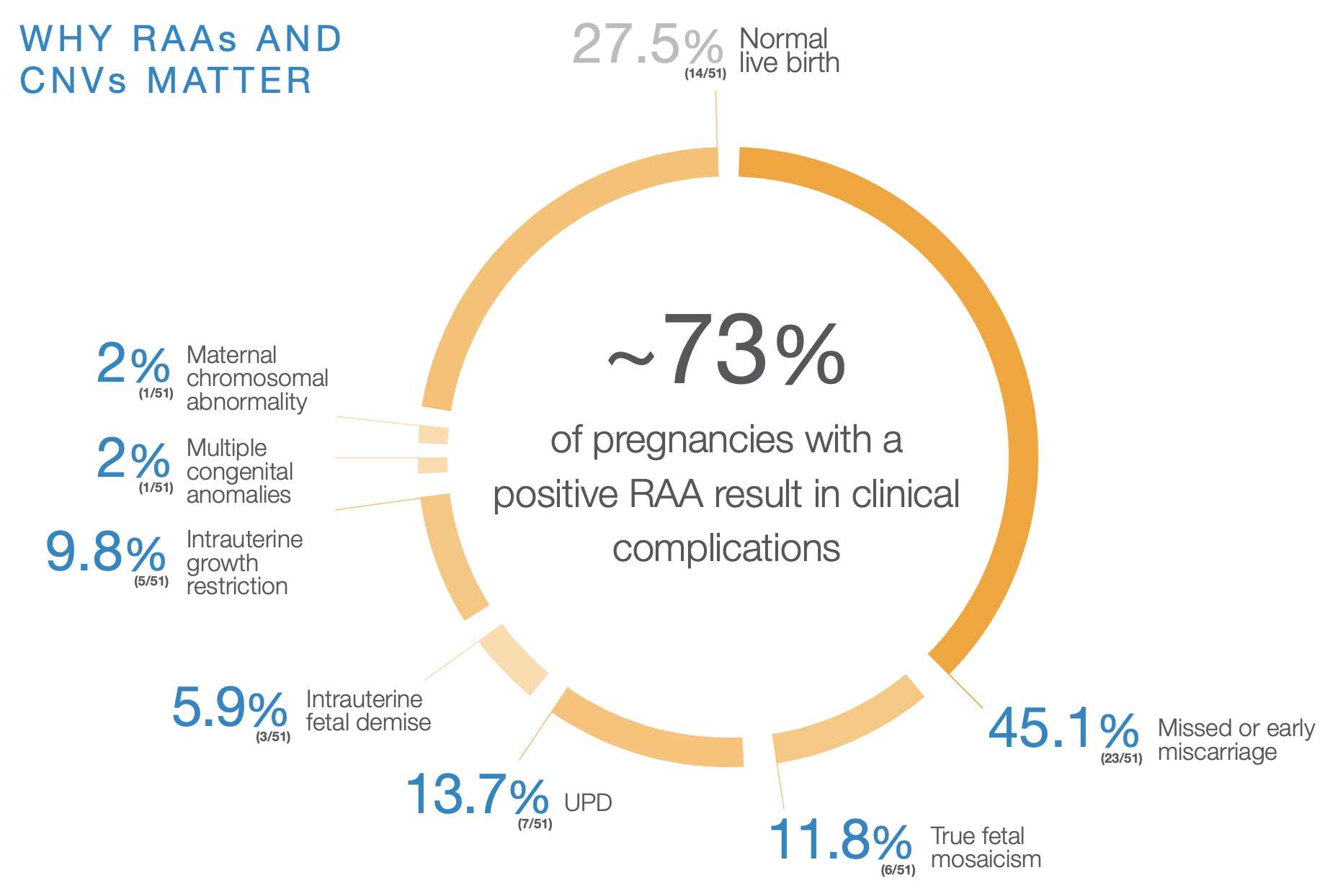
RAAs were detected using expanded NIPT in 51 cases. Cases may fall into more than one category, therefore the total may exceed 100%. This study included only rare autosomal trisomies. The screen positive rate for all RAAs, which also includes monosomies, is expected to be marginally higher than the rate reported for rare autosomal trisomies.
CNV=copy number variation; RAA=rare autosomal aneuploidy; UPD=uniparental disomy.
SUPERIOR PERFORMANCE, MORE CONFIDENCE
A positive RAA or CNV NIPT result can represent the fetal and/or placental genome. Either way, a positive result indicates an increased risk for fetoplacental disease and is associated with clinical relevance in half of the cases. Even if the RAA or CNV is mosaic in nature, an abnormality within the fetus and/or placenta may manifest itself.
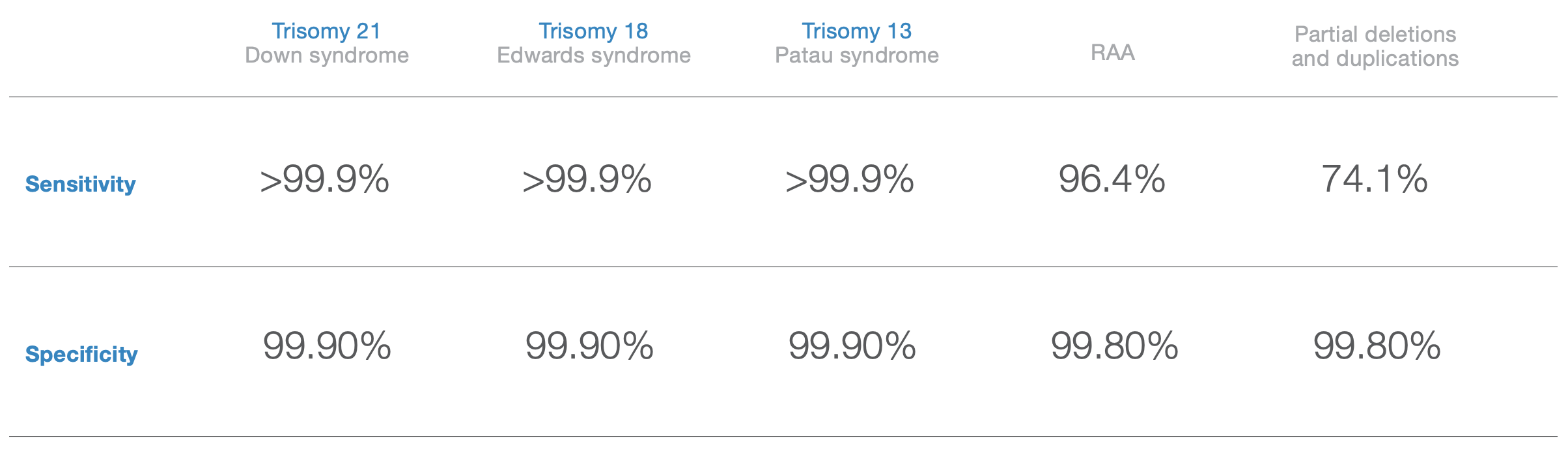
Disclaimer: Sensitivity and specificity of the VeriSeqTM NIPT Solution v2 for detecting trisomies 21, 18, and 13 in a basic screen for singleton pregnancies (excluding known mosaics).
Source: VeriSeqTM NIPT Solution v2 package insert, April 2020. Data on file, Illumina, Inc. 2020.
WHAT DOES A POSITIVE RESULT MEAN?
It is important to note that NIPT24 is a screening test, not a diagnostic test. Any positive result needs to be confirmed with diagnostic testing. NIPT results should not be used as the sole basis for pregnancy management decisions.
Further testing and evaluations are appropriate, and potential clinical implications. Testing options can include (but are not limited to):

Detailed ultrasound evaluation to confirm fetal viability, identify possible structural anomalies in the fetus, and/or to determine if the pregnancy is at risk for complications such as intrauterine growth restriction
Diagnostic testing via chorionic villus sampling or amniocentesis to determine if the positive NIPT result is indicative of a true fetal chromosomal abnormality, fetal mosaicism, fetal uniparental disomy, or placental mosaicism
Additional specialized testing (in some cases)
RESOURCES FOR HEALTH CARE PROVIDERS
Scientific Validation Studies
- Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing.
- DNA sequencing versus standard prenatal aneuploidy screening.
- Copy-Number Variation and False Positive Prenatal Screening Result.
Clinical Experience Studies
- Noninvasive prenatal testing in the general obstetric population: clinical performance and counseling considerations in over 85 000 cases.
- Aneuploidy screening by non-invasive prenatal testing in twin pregnancy.
- Fetal sex chromosome testing by maternal plasma DNA sequencing: clinical laboratory experience and biology.
VeriSeq NIPT Solution v2 Publications
- Analysis of cfDNA in a consecutive series of 13,607 routine samples
- Strategy for use of genome-wide non-invasive prenatal testing
- Clinical experience with noninvasive prenatal testing in Germany: analysis of over 500 high-risk cases for trisomy 21, 18, 13 and monoso
Genome-Wide Publications
- Origin and clinical relevance of chromosomal aberrations other than the common trisomies detected by genome-wide NIPS: results of the TRIDENT study
- Rare autosomal trisomies, revealed by maternal plasma DNA sequencing, suggest increased risk of feto-placental disease
- Rare autosomal trisomies: Important and not so rare
- Outcome of publicly funded nationwide first-tier noninvasive prenatal screening
Other Papers of Interest ( Diğer Yayınlar)
- Current use of noninvasive prenatal testing in Europe, Australia and the USA: A graphical presentation
- Fetal fraction and noninvasive prenatal testing: What clinicians need to know
